Infusion Pump Testing Support
Advice and support for users of Rigel’s infusion pump analyzers
Get the most out of your Rigel infusion pump analyzers and find the answers to your questions with our FAQs.
What is infusion pump testing?
An infusion pump is an electronic device used to control the administration of intravenous fluids to deliver measured amounts at careful and regulated rates. They can produce high and low but controlled pressures to inject controlled amount of fluids.
The reliability of infusion devices is important as these devices are very common, as well as being used on patients who are likely to be in a critical condition. There is a wide range of methods used to test the performance and accuracy of infusion devices which vary in procedure and equipment. The primary aim is to accurately measure the delivery volume and flow rate of the infusion device, check occlusion alarms and determine that it is safe for use.
Testing is provided in the manufacturer’s preventative maintenance procedure and ensures that the equipment is working within its specification and is fit for purpose. The primary test is to measure the accuracy of the delivery volume and flow rate over a range of time periods, typically between 10 minutes and 1 hour.
Occlusion tests check the capability of an infusion device to detect an obstruction. With a blockage, pressure will build up and it’s important that an infusion device will activate an alarm when specific pressure thresholds have been exceeded.
Bolus delivery must also be tested to maintain the performance of the infusion device especially with patient controlled devices where the bolus is self-medicated.
Why test infusion pumps?
Infusion devices are used extensively in clinical conditions and patients’ homes providing perioperative care, critical care and pain management. It is estimated that 80% of hospitalised patients receive intravenous (IV) therapy. Therefore, it is essential that the infusion of fluids to a patient is in an accurate predetermined and consistent manner. There is a variety of infusion device methods which provide the ability to feed, hydrate, medicate or replace blood loss to a patient.
Millions of infusion devices are safely used in hospitals and in the community every year. Incidents with infusion devices continue to dominate adverse incident reports with at least 1000 incidents investigated by the MHRA between 2005 and 2010 in the UK alone. The majority of problems relate to over-infusion of drugs, either due to user error with dosage and patient data, product design and engineering or software malfunction. However, genuine infusion pump malfunctions rarely occur, except when a pump has been mishandled, dropped or damaged.
Infusion devices must be capable of delivering infusions at pressures between 100 and 750mmHg (2 to 15PSI) to overcome any internal or external resistance to the flow of fluid. Resistance arises due to filters, anti-siphon and anti-reflux valves, administration set, in particular the cannula, the internal diameter, potential kinking of tubing, sticky (viscous) solutions and syringe/cassette stiction can accumulate the pressure. The patient’s venous system contains pressure and therefore the IV infusion device must be proven to be compatible with pressures found in the body.
How do I perform an occlusion test on B. Braun Space Series, Infusomat and Perfusor devices?
This document has been produced to aid in testing the B. Braun infusion pumps for the Space series, Infusomat and Perfusor occlusion performance. The B Braun pump has a unique auto rewind function when occlusion pressure limit is released to relieve the pressure and then continue to infuse when the pressure has been reduced.
Equipment required
- B Braun Pump and giving set
- Multi-Flo Infusion device
Optional:
- Med-eBase software for remote control (High resolution real time graphs)
1) Connect the Rigel Multi-Flo and B Braun infusion device
2) If using Med-eBase; connect the Multi-Flo to the PC using a USB cable and load Med-eBase V2.4 (or higher) software and open the remote control dashboard.
Note: Please see the Med-eBase manual for setting up the Multi-Flo to the PC software.
3) The occlusion test needs to be set up in Manual mode
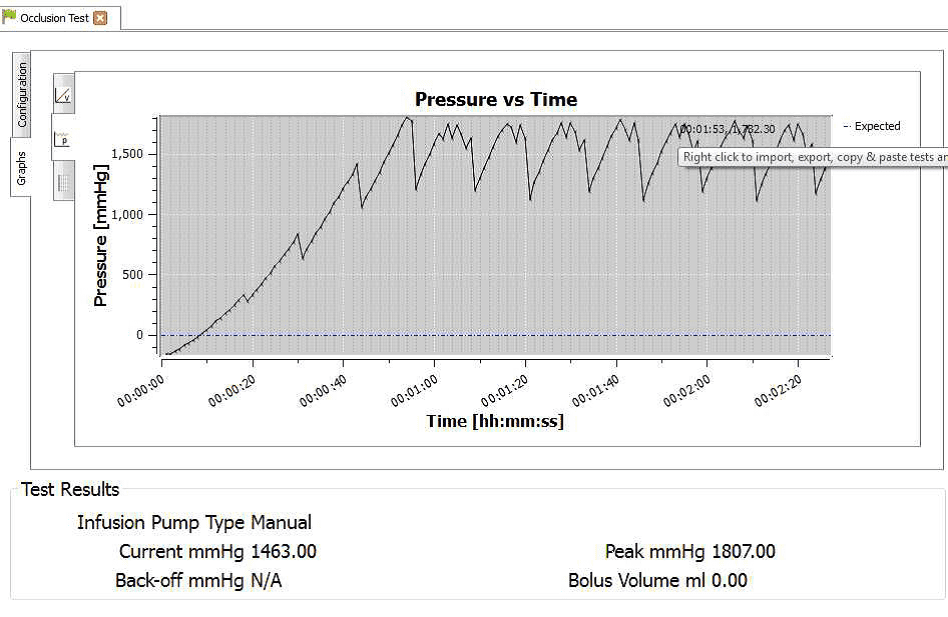
4) When the occlusion alarm sounds DO NOT press the green button on the Multi-Flo. This will allow the Braun device to reverse the pressure and begin to pump again. The user should end up with an image on screen or on the PC as follows:
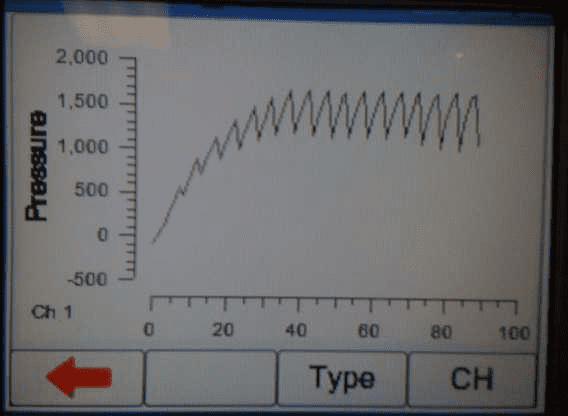
5) Once enough results have been captured press the green button to stop the test or select manual stop in the software
6) If using the Multi-Flo as a standalone device in manual mode go back to the summary screen and select SAVE (F2); in
AUTO MODE these results save automatically. Download the results into Med-eBase using the USB cable. Detailed instructions on how to download can be found in the Med-eBase manual.
7) From Med-eBase the results can be viewed by searching for the Asset ID of the device. Select the TEST RESULTS tab and select the test. On the test results scroll down to the graph section and select the pressure graph.
8) Hover the mouse on the maximum and minimum points to record the pressure readings at each point.
9) Alternately the results can be exported as a CSV file fromMed-eBase to a location of your choice for further analysis.
Med-eBase remote control Multi-Flo
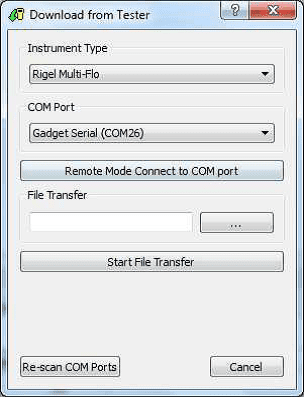
1) Open Med-eBase and select Tools > download. Select the gadget serial to open the Multi-Flo remote control dashboard.
2) Once the software is opened in remote control. Set up the occlusion test as a Manual Occlusion by select the correct channel and then Add Occlusion test. Select Start test and assign the test to an Asset ID.
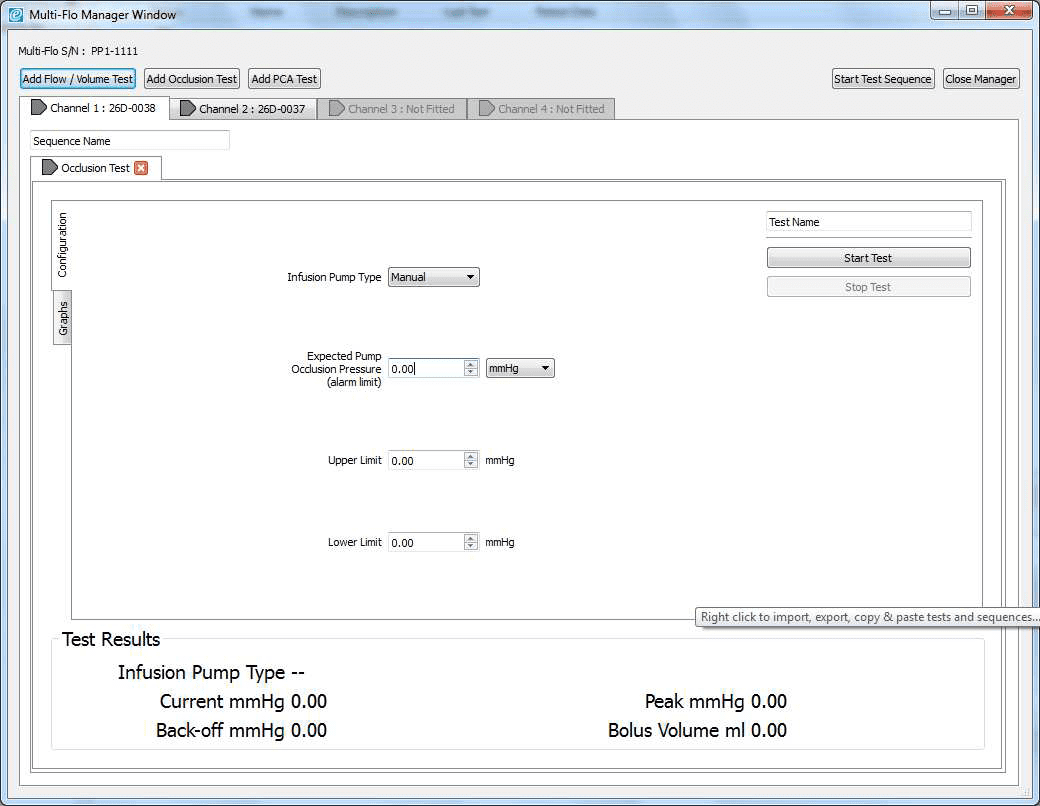
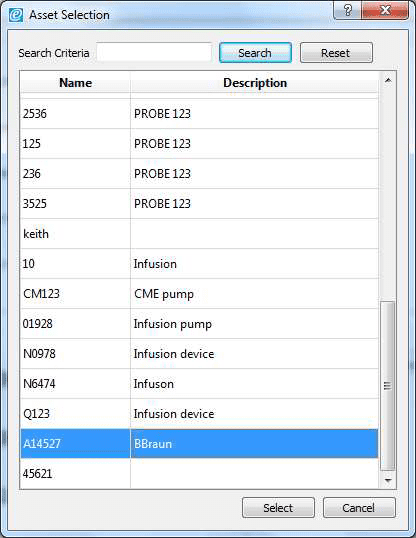
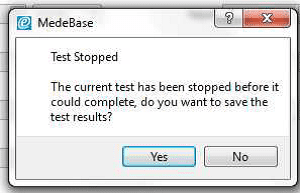
3) Allow the B Braun pump to reach the peak pressure limit a number of times before ending the test by selecting Stop Test in the Configuration tab. A user message will appear indicating that the test has stopped and to ask whether to save the results. Select Yes and the results are automatically saved to the Asset ID select at the start of the test.
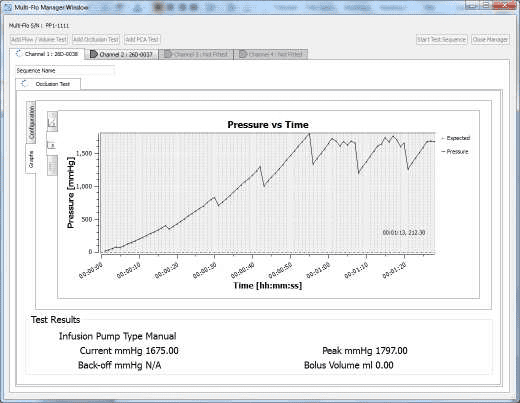
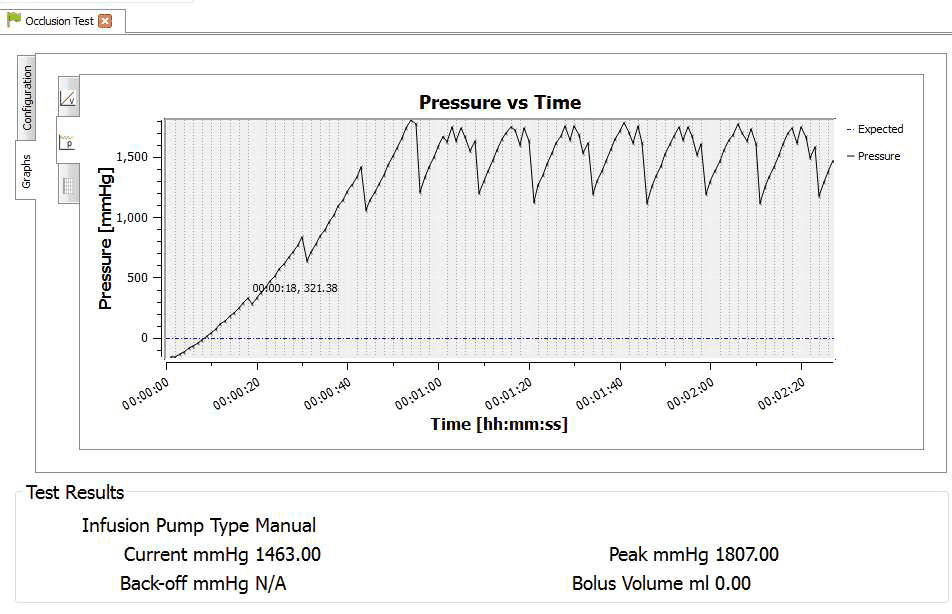
4) Once the test is complete the user can hover the mouse over specific points and record readings. From the service manual the pressure stages and ranges are indicated below:
Pressure stage 1 range 75 – 450 mmHg
Pressure stage 5 range 300-750 mmHg
Pressure stage 9 range 600 – 1050 mmHg
Example:
Pressure stage 1: 321mmHg
Pressure stage 5: 624 mmHg
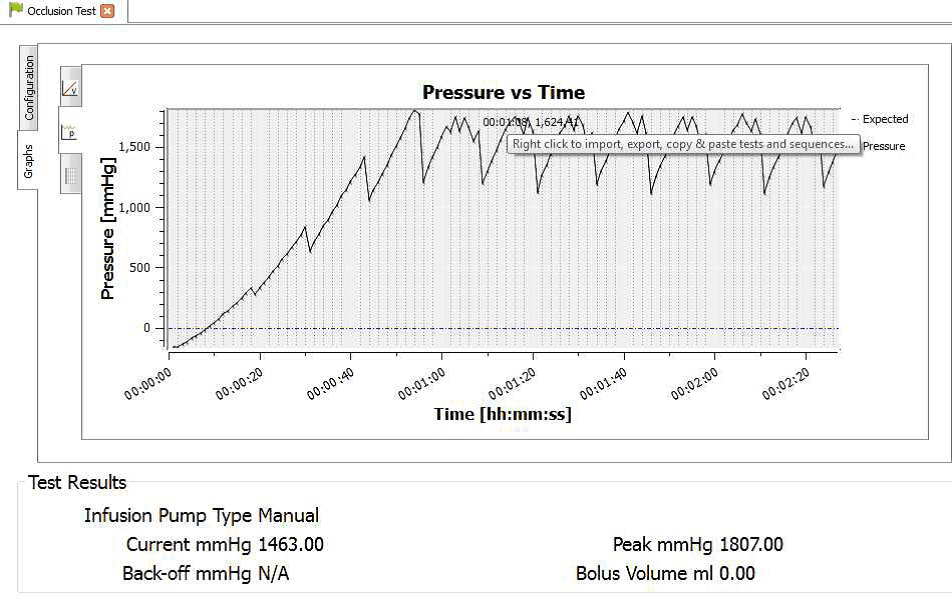
Pressure stage 9: 782mmHg
How to test infusion pumps?
The IEC 60601-2-24 standard states the performance and usability requirements for infusion devices. There is a wide range of methods used to test the performance and accuracy of infusion devices which vary in procedure and equipment. The primary aim is to accurately measure the delivery volume and flow rate of the infusion device, check occlusion alarms and determine that it is safe for use.
Testing is based on manufacturer recommendation in their performance procedure. This ensures that the equipment is working within its specification and is fit for purpose. The accuracy of the whole system, not just the device, must be included for infusion device testing. Possible inaccuracies can occur because of the syringe used and tubing.
Infusion device analysers offer an all in one solution for testing. They record real-time readings of the delivery rate and volume allowing for continuous infusion device testing without constant supervision. Some analysers have up to 4 channels, which can run simultaneously and even measure boluses. Furthermore, occlusions in the pressure circuit can be simulated by the analyser to test the infusion devices occlusion alarms.
Sign up to our mailing list today to stay up to date with the latest industry news and information from Seaward.